WESTERN PRODUCER — It’s the cold cloud with freezing precipitation that dominates our weather for most of the year on the Canadian Prairies.
Even in the summer, most of the precipitation-forming clouds fall into this category.
For atmospheric water to freeze, it must have something to freeze onto, in the same way that water droplets need something to condense onto.
The problem that arises is that in the atmosphere, there are large numbers of particles for water to condense onto (condensation nuclei) but very few particles for water to freeze onto (ice nuclei).
For ice to form (at temperatures just below zero), you need a six-sided structure, and there are not many of these around.
Ice crystals themselves are six-sided, but where do you get the ice crystal in the first place?
Because of this, if the cloud temperature is warmer than –4 C, the cloud will be made up of super-cooled water. If we cool the cloud down to around –10 C, ice crystals will begin to form even if there are no ice nuclei, so at these temperatures the cloud will consist of a mixture of ice crystals and super-cooled water.
The super-cooled water that falls from these clouds will freeze as soon as it hits a cold surface. Once temperatures fall to –30 C, the cloud will consist almost entirely of ice crystals, and if we are colder then –40 C, the whole cloud will be made up of ice crystals.
OK, so now we know that within cold clouds we will usually have a combination of ice crystals and super-cooled water. How does this tie into the creation of precipitation in cold clouds?
That’s where the Bergeron process comes into play, which relies on another unique property of water — if there is just enough water vapour in the air to keep a super-cooled water droplet from evaporating, then there is more than enough water vapour in the air for an ice crystal to grow larger.
Because the saturation vapour pressure over ice is lower than that over water, ice crystals will attract water vapour more readily than water droplets will.
Our cold cloud now has ice crystals in it, and these ice crystals are growing. As the crystals grow, they pull water vapour from the atmosphere.
As the amount of water vapour in the atmosphere drops, our super-cooled droplets will begin to evaporate to help make up the difference. These droplets evaporate, and the ice crystals continue to grow at the expense of the super-cooled water droplets.
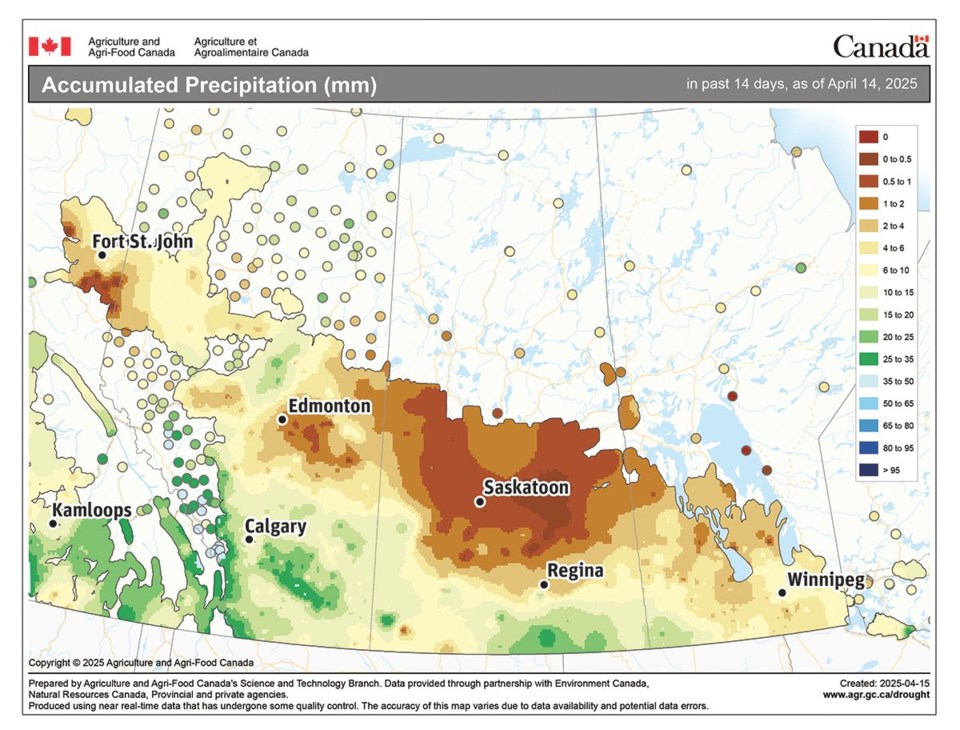
April has started off dry across the northern parts of the agricultural Prairies with the driest weather occurring over central Saskatchewan. Parts of southern Alberta and extreme southern Saskatchewan have seen the most precipitation with some regions reporting between 25 and 50 millimetres.
After a while, the cloud consists mostly of ice crystals. This process by itself would only result in light amounts of precipitation, although for heavier precipitation, we need the second process to kick in. In a cold cloud, we call this second process riming and aggregation.
As ice crystals fall, they collide into either super-cooled water and grow larger (riming), or they collide into other ice crystals and grow larger (aggregation).
Aggregation occurs best when cloud temperatures are only slightly below zero because the warmer temperatures allow the ice crystals to have a wet surface that helps other ice crystals stick to them. This is one of the reasons we see large snowflakes when it is relatively warm.
In the next issue, we’ll continue our discussion by looking at different forms of precipitation.
Daniel Bezte is a teacher by profession with a BA in geography, specializing in climatology, from the University of Winnipeg. He operates a computerized weather station near Birds Hill Park, Man. Contact him at [email protected].
About the author
Related Coverage




